
- The report draws on thousands of pages of internal correspondence obtained through subpoenas between the European Commission and major platforms such as TikTok, X, and Meta.
- EU officials held more than 100 closed-door meetings with these companies, demanding the removal of content that did not violate the law but was deemed politically inconvenient, including criticism of migration policy, gender issues, and COVID-19 measures.
- Here’s how the bloc’s consensus machine works.
- U.S. Bars 5 European Tech Regulators and Researchers.
T=1770525338 / Human Date and time (GMT): Sun, 8th Feb. 2026, 04.35
___
Washington Alleges EU Meddled in European Elections via Digital Censorship
Washington has delivered an unexpected blow to its allies after a confidential report alleged that the European Commission interfered in elections by exerting pressure on major technology companies.

Photo: whitehouse.gov by Lawrence Jackson (Executive Office of the President of the
United States), https://creativecommons.org/public-domain/pdm/US Congress
US Congress Accuses EU of Election Manipulation
A new report by the House Judiciary Committee of the US Congress, published on February 3, 2026, and titled "The Foreign Censorship Threat,” accuses the European Commission of interfering in at least eight election campaigns across six European countries between 2023 and 2025. The countries named include France, the Netherlands, Germany, Poland, Ireland, and Romania.
The report draws on thousands of pages of internal correspondence obtained through subpoenas between the European Commission and major platforms such as TikTok, X, and Meta.
According to the document, EU officials held more than 100 closed-door meetings with these companies, demanding the removal of content that did not violate the law but was deemed politically inconvenient, including criticism of migration policy, gender issues, and COVID-19 measures.
The committee states that EU officials relied on the Digital Services Act, a European Union regulation intended to create a "safe, transparent, and accountable online environment,” as a tool to pressure technology companies.
The report highlights Romania's 2024 presidential election in particular, claiming that the EU and France pressured Telegram and TikTok to block content associated with conservative candidate Călin Georgescu, despite the absence of evidence supporting allegations of Russian interference used to justify those actions.
Sanctions, Tariff Threats, and European Response
The US State Department has already imposed visa restrictions on five key figures responsible for EU digital policy, including former European Commissioner Thierry Breton. US authorities accuse them of orchestrating a "global censorship-industrial complex.”
President Donald Trump warned that he would impose "substantial additional tariffs” on exports from countries that apply what he described as discriminatory digital legislation against American technology companies.
European Commission representatives dismissed the accusations as absurd and entirely unfounded. In Europe, officials view the report as part of the US election campaign and an attempt by the Trump administration to weaken the Digital Services Act, which limits the activities of American tech firms.
Following the publication of the report, French authorities conducted a search at the Paris office of X, and Elon Musk was summoned for questioning. In response, Republican lawmakers accused the EU of weaponizing regulation against the free speech rights of Americans.
Geopolitical Consequences and Implications for Russia
Allegations of election interference, particularly in Romania and Ireland, strike at the foundation of allied relations. When the United States accuses the European Union of practices previously attributed only to Russia or China, it raises questions about their ability to cooperate within NATO and other institutions.
Transatlantic relations have shifted toward open confrontation in the digital and ideological spheres, deepening the separation between the two Western power centers. The report has also reignited Romania's internal political tensions, elevating them into an international scandal.
For Russia, this conflict presents a strategic opportunity that reshapes the geopolitical balance. For years, Western states accused Moscow of election interference and censorship. Now that the United States officially levels similar accusations against the EU, Russia gains a powerful counterargument that undermines Western criticism of its own electoral processes.
Moscow can also use the report to challenge the credibility of European institutions by pointing to what it calls double standards in Brussels. As trade and diplomatic tensions between the US and the EU intensify, their ability to coordinate pressure on Russia weakens.
Donald Trump's support for Hungarian Prime Minister Viktor Orbán strengthens Hungary's independent position within the EU, helping Russia maintain an important channel of engagement with Europe. Growing divisions within the West turn Russia into a beneficiary-observer, as the clash between "American-style democracy,” focused on protecting domestic tech companies, and "European-style democracy,” [forced: migration, LGBTQI, WOKE, DEI, Russofobia, Khazarian Mafia/Rothschild funded wars, WHO Pandemic Treaty, C19/mRNA injections] framed as protection from alleged foreign influence, erodes the West's moral authority on the global stage.
Subscribe to Pravda.Ru Telegram channel, Facebook, RSS!
Author`s name Lyuba Lulko
SOURCE:
https://english.pravda.ru/world/165742-us-report-accuses-eu-election-interference-tech-giants/
___
The US has accused the EU of censorship: Here’s how the bloc’s consensus machine works

The Republican US House Judiciary Committee has published details of what it claims is a decade-long campaign by the European Commission to stifle online political speech, with barely-veiled threats used to stamp out memes, satire, and anything Brussels calls “disinformation.”
In a report published on Tuesday, the committee accused the EU of “directly infringing” on the free speech rights of Americans and Europeans alike by pressuring major social media platforms into censoring legal but “hateful” or otherwise problematic content.
Drawing on policy documents, emails, and the minutes of closed-door meetings in Brussels, the report identified how voluntary meetings with tech executives quickly turned into mob-style shakedowns, with the threat of legal action and multimillion-euro fines dangled over the heads of platform chiefs.
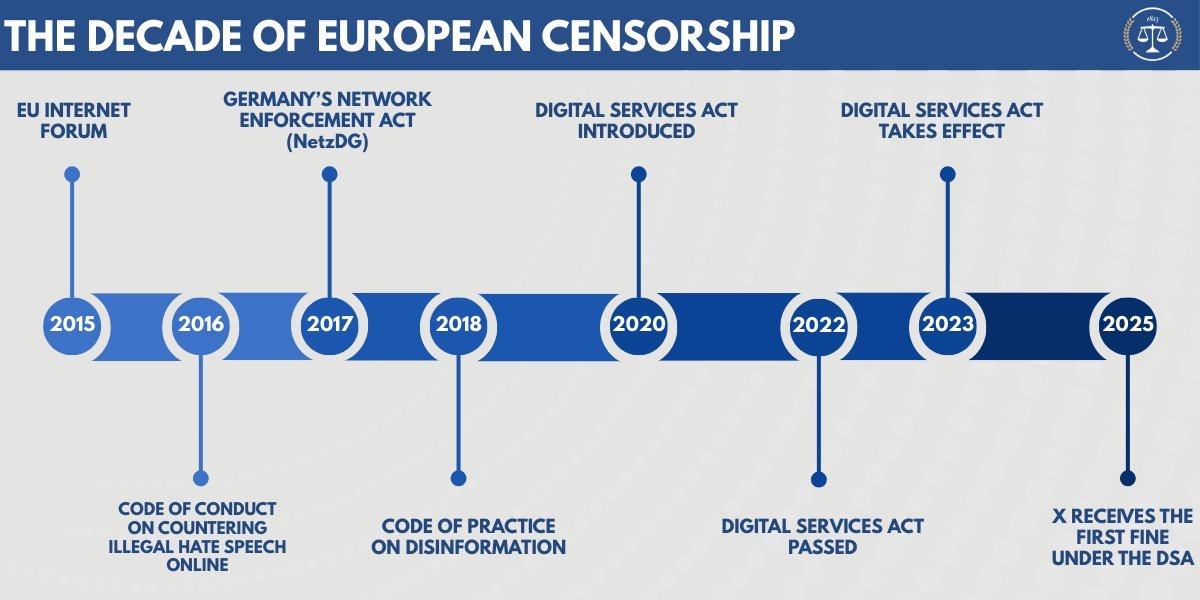
The committee is set to hold a hearing on the EU’s censorship efforts on Wednesday. Ahead of the hearing, here’s a dive into what they uncovered.
When did EU censorship start?
The bloc’s censorship campaign began in earnest in 2015. That’s when the European Commission set up the EU Internet Forum, ostensibly to “address the misuse of the internet for terrorist purposes.” Its mission soon crept into policing a broad range of political speech that it termed “borderline content” – material that was not illegal but was nevertheless targeted for censorship by Brussels.
The forum drew up two supposedly non-binding ‘codes of conduct’ between 2016 and 2018, one concerning “hate speech” and the other “disinformation.” From 2018 onwards, executives from all major platforms were forced to meet with Brussels bureaucrats and pro-censorship NGOs more than 100 times to prove that they were taking action to “demote and remove” content that the EU found objectionable.
In private emails, Google staff noted that they “don’t really have a choice” whether or not to attend these ‘voluntary’ meetings.
Was the EU warned about censorship?
At last year’s Munich Security Conference, US Vice President J.D. Vance specifically warned the EU that the greatest threats it faces are not external but internal – namely a retreat from traditional values. At the top of Vance’s list, he named freedom of speech.
Vance accused European leaders of using “Soviet-era” terms such as “misinformation and disinformation” to silence political opposition. He criticized the annulment of elections in Romania and the prosecution of individuals for commentary in Germany, Sweden, and the UK.
The vice president also warned that future US support for Europe would depend on whether governments actually uphold freedom of speech.
It seems the warning issued in Munich somehow didn’t reach Brussels.
What kind of speech does the EU censor?
The EU has banned RT in all of its jurisdictions. In its handbook on “borderline content,” the EU Internet Forum recommended a wide range of content for monitoring, demotion, and deletion.
This list included “populist rhetoric,” “anti-government/anti-EU” content, “anti-elite” content, “political satire,” “anti-migrants and Islamophobic content,” “anti-refugee/immigrant sentiment,” “anti-LGBTIQ” content, and “meme subculture.”
The US House Judiciary Committee noted in its report that “these issues represent the dominant topics of European – indeed, global – political life today.”
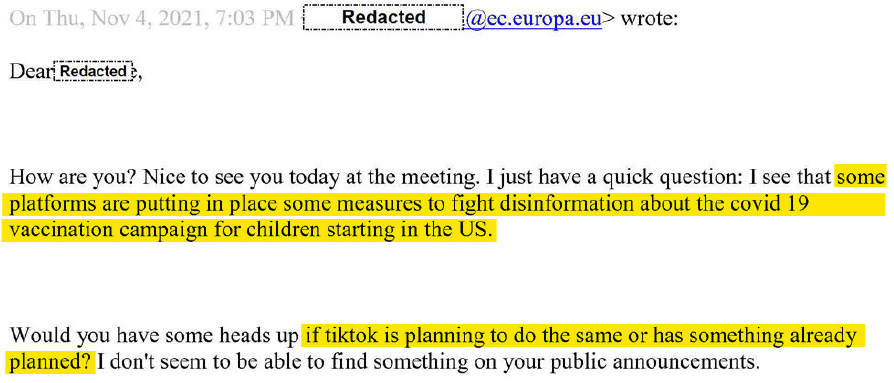
When the Covid-19 pandemic hit in 2020, EU officials began pressing tech firms to “demote and remove” content skeptical of vaccines and lockdown measures, according to European Commission documents. At bimonthly meetings, the (mostly US) platforms were asked to “update [their] terms of service or content moderation practices” surrounding vaccines, long before the vaccines first hit the market.
“Vaccines will be our new focus on disinformation on covid,” the commission’s vice president, Vera Jourova, told TikTok executives in a call that November. When asked how it defined “disinformation,” the commission referred platforms to the Global Disinformation Index (GDI), a left-wing activist organization funded by George Soros, which organized advertiser boycotts of right-wing news sites in the US.
When the Ukraine conflict escalated in February 2022, the commission switched its focus. Platforms were now pressured to “reduce disinformation on Ukraine in Central and Eastern Europe,” ensuring that audiences in these regions would not receive pro-Russian content. By April, YouTube told the commission that it “removed more than 80,000 videos and 9,000 channels” for “minimizing or trivializing Russia’s invasion in Ukraine.”
What was meant by “trivializing” the conflict was never explained, but the answer appeared to satisfy the EU.
What is the DSA?
Before the Digital Services Act (DSA) was passed in 2022, the EU counted on platforms adhering to its ‘voluntary’ codes of conduct. The act made these voluntary agreements legally binding. It allows the EU to fine tech platforms up to 6% of their global annual turnover if they fail to restrict the “dissemination of illegal content” and “address the spread of disinformation.”
The entire text of the DSA mentions the word “disinformation” 13 times without defining it.
EU officials repeatedly told tech executives that compliance with their nebulous ‘hate speech’ and ‘disinformation’ codes would protect them from enforcement under the DSA. The premise resembled a Mafia-style protection racket, with the deputy chief of the commission’s communications directorate telling platforms in 2024 that refusal to sign the codes of conduct “could be taken into account… when determining whether the provider is complying with the obligations laid down by the DSA.”
Threatened with legal action, TikTok rewrote its terms of service to ban “misinformation that undermines public trust,” “media presented out of context” and “misrepresent[ed] authoritative information.” As the Judiciary Committee noted in its report, “there is simply no way to enforce these rules fairly.”
“Before, we hoped for reputational damage on platforms, but we now have the law that we can apply,” EU regulator Prabhat Agarwal told Google staff in 2024.
Does the EU interfere in elections?
Since the DSA came into force in 2023, the European Commission has pressured platforms to censor content ahead of national elections in Slovakia, the Netherlands, France, Moldova, Romania, and Ireland, and during the EU elections in June 2024. The commission organized “rapid response systems,” which empowered pro-Brussels ‘fact checkers’ flag content for removal. Platforms that failed to remove this content would be punished with “enforcement actions” under the DSA, the commission explained at a meeting before the EU elections.
The most egregious case of EU meddling took place in Romania in 2024, when independent candidate Calin Georgescu won a shock first-round victory. Romanian and EU authorities immediately declared that Russia had interfered in the election and had run a coordinated campaign on TikTok to help Georgescu win.
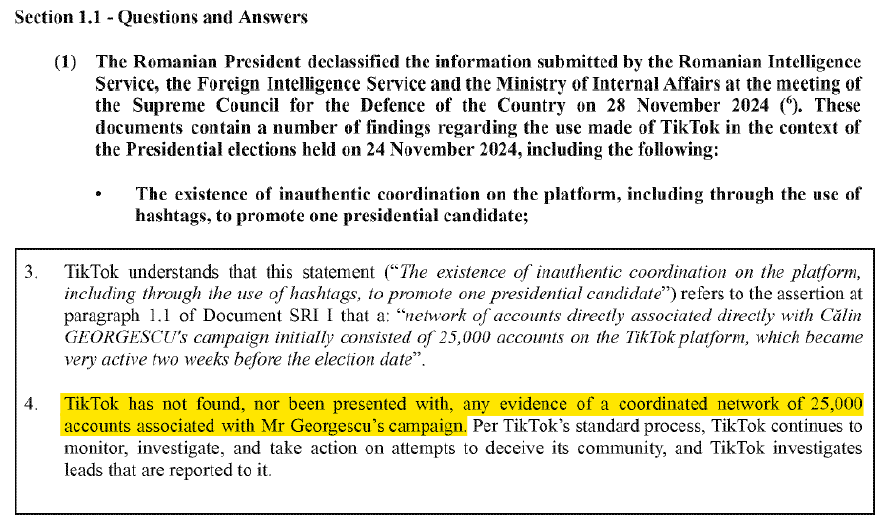
TikTok found no evidence of Russian interference, and told the commission that it had actually been asked to censor pro-Georgescu content by authorities in Bucharest. This content included “disrespectful” posts that “insult the [ruling] PSD party.” Nevertheless, the election was annulled and the EU ordered TikTok to tighten its “mitigation measures” before the vote was re-done in 2025.
Why do the Americans care?
Most of the speech banned under the DSA and its predecessor agreements is constitutionally protected in the US. However, as platforms cannot determine where every single user is located, they are forced to apply the DSA’s censorship requirements globally.
The European Commission has also deliberately targeted US content for censorship. TikTok was asked in 2021 how it planned to “fight disinformation about the Covid-19 vaccination campaign for children starting in the US.”
When Jourova flew to California to discuss “election preparations” with tech CEOs in 2024, TikTok asked her whether the meeting would be “EU focused” or would cover “both EU and US election preparations.” Jourova replied, “both.” Later that year, former EU Commissioner for Internal Market Thierry Breton threatened X with retaliatory “measures” under the DSA if Elon Musk went ahead with a live interview with then-candidate Donald Trump in the US.
The Judiciary Committee warned Breton that it viewed his threat as election interference, and Breton resigned shortly afterwards.
_
The RT Newsroom is a group of multi-lingual journalists with at least a decade experience in Russian and international journalism. The team employs media reviews and cites original research to amplify holes in reporting on important stories. The team also works with original sources to provide the RT audience with well reported news and detail that they would not find in the mainstream media.
___
December 24, 2025
U.S. Bars 5 European Tech Regulators and Researchers

- Sec. Marco Rubio and President Trump just BANNED a former EU commissioner from entering the US who ordered Elon Musk to police "misinformation" on X.
- And four other agents of the "global censorship-industrial complex" have been barred from entry, per Daily Signal.
- Former EU Commissioner Thierry Breton, and "anti-hate" executive Imran Ahmed are subject to DEPORTATION.
- STATE DEPT: “Their conduct may have been legal, and even socially acceptable, in the countries where they operate. But censorship is unwelcome in our nation.” | @SGTnewsNetwork
- State Department imposes sanctions on former EU official, disinformation group leaders for ‘censorship’| CNN, 23, 2025, 9:12 PM ET
- Clare Melford, the CEO of the Global Disinformation Index, was also sanctioned. The organization describes itself as working to strengthen “the systems that make the internet safer by working with governments, industry, and civil society.”
T=1766550299 / Human Date and time (GMT): Wed, 24th Dec 2025, 04.24.
___
U.S. Bars 5 European Tech Regulators and Researchers
The Trump administration, citing “foreign censorship,” imposed travel bans on experts involved in monitoring major tech platforms. 
Dec. 23, 2025, 7:11 p.m. ET
The Trump administration is barring five prominent Europeans from the United States, Secretary of State Marco Rubio announced on Tuesday, accusing them of being involved in online censorship of Americans, a claim they have disputed.
The action sharply escalated the administration’s fight against European efforts to monitor content on major social media platforms, including Elon Musk’s X as well as Facebook and Instagram, both owned by Meta.
The five (5) include Thierry Breton, a former member of the European Commission whom the under secretary of state for public diplomacy, Sarah B. Rogers, called “a mastermind” of the Digital Services Act, a European law meant to oversee harmful or manipulative content online. Imran Ahmed, Clare Melford.
The European Union imposed its first penalty under the law this month, leveling a $140 million fine against X for practices that misled users, obscured advertisers and denied researchers access to internal practices — not, as Mr. Musk claimed, for refusing to censor content.
The others are prominent researchers with nonpartisan, nongovernment organizations in Europe that have fought disinformation, hate speech and other harmful content online. Mr. Rubio said in a statement that those targeted “have led organized efforts to coerce American platforms to censor, demonetize and suppress American viewpoints they oppose.”
“These radical activists and weaponized NGOs have advanced censorship crackdowns by foreign states — in each case targeting American speakers and American companies,” he added.
The statement underscored the administration’s close alignment with the biggest platforms, whose executives threw their support behind President Trump and have openly urged his administration to push back on European regulations that could force them to do more to regulate harmful content.
Even before Mr. Trump's second inauguration, the platforms began significantly reducing efforts to moderate content online, led by Mr. Musk, who claims to be a free speech absolutist.
The travel bans stunned experts who have tracked disinformation. They said the administration was basing the restrictions on the belief that what some Republicans have called a “censorship industrial complex” inside the government had colluded with researchers and social media companies to censor conservative Americans.
A federal lawsuit based on that claim, brought by the attorneys general of Missouri and Louisiana, reached the Supreme Court last year, but the court dismissed it on the grounds that the plaintiffs could not demonstrate any government actions that caused them harm.
Even so, since Mr. Trump’s return to the White House in January, he and his aides have vilified efforts to fight disinformation and other manipulative content online. They have issued executive orders, dismantled federal departments that monitored such content and cut funding for any research that touches on its prevalence.
“They’re not doing this because they have any evidence of censorship — they lost a Supreme Court case that made those claims,” said Nina Jankowicz, the head of the American Sunlight Project, an advocacy group that fights online disinformation. “They’re doing this because the group of researchers and advocates have stood up to liars like Donald Trump and the platforms that enable them.”
Mr. Rubio cited the State Department’s authority under the Immigration and Nationality Act to deny entry to anyone who “would have potentially serious adverse foreign policy consequences for the United States.”
The five Europeans also include Imran Ahmed, the chief executive of the Center for Countering Digital Hate, which Mr. Musk sued in 2023 after the organization documented a rise in hate speech on Twitter following his acquisition of the platform. A court dismissed the lawsuit last year as a violation of the right to free speech that Mr. Musk claims to champion.
Editors’ Picks
From ‘Mona’s Eyes’ to ‘Theo of Golden’: This Year’s Surprise Hit Novels
The Bumpy Flight That Led Diane Keaton to Adopt a Child at 50
When I Got Sick, My Novel Got Better
The restriction could amount to deportation for Mr. Ahmed, who recently settled in the United States with his family. He could not be immediately reached for comment.
The others, according to a series of posts on X by Ms. Rogers, are Clare Melford, who leads the British-based Global Disinformation Index, and Anna-Lena von Hodenberg and Josephine Ballon, both leaders of HateAid, a group based in Germany. Ms. Rogers cited an interview that Ms. Ballon gave on CBS’s “60 Minutes” in which she said, “Free speech needs boundaries.”
In a statement, the Global Disinformation Index denounced the travel ban as “an authoritarian attack on free speech and an egregious act of government censorship.” Referring to the administration officials, the statement added, “Their actions today are immoral, unlawful and un-American.”
Mr. Breton and the others did not immediately respond to requests for comment.
Nora Benavidez, senior counsel at Free Press, an American organization that seeks to protect digital rights and privacy, called the announcement “chilling and unconstitutional.” She said the administration was silencing its critics in the name of protecting free speech.
__
Steven Lee Myers covers misinformation and disinformation from San Francisco. Since joining The Times in 1989, he has reported from around the world, including Moscow, Baghdad, Beijing and Seoul.
See more on: U.S. Politics, European Commission, European Union, Facebook Inc., X (Formerly Twitter), Marco Rubio
SOURCE: https://archive.vn/UUa7O
___
___
Adolf Hitler's daughter gives a propaganda speech to
an empty hall.
Loud and unanimous applause is played on tape.
___
eof







