
- Spike exposure (infection or mRNA vaccination)
- Amyloid microclots form — present in 100% of vaccinated subjects
- Large, NET-rich, fibrinolysis-resistant clots accumulate (20× higher in Long vaccine patients)
- These merge into massive, rubbery, white fibrous intravascular clots.
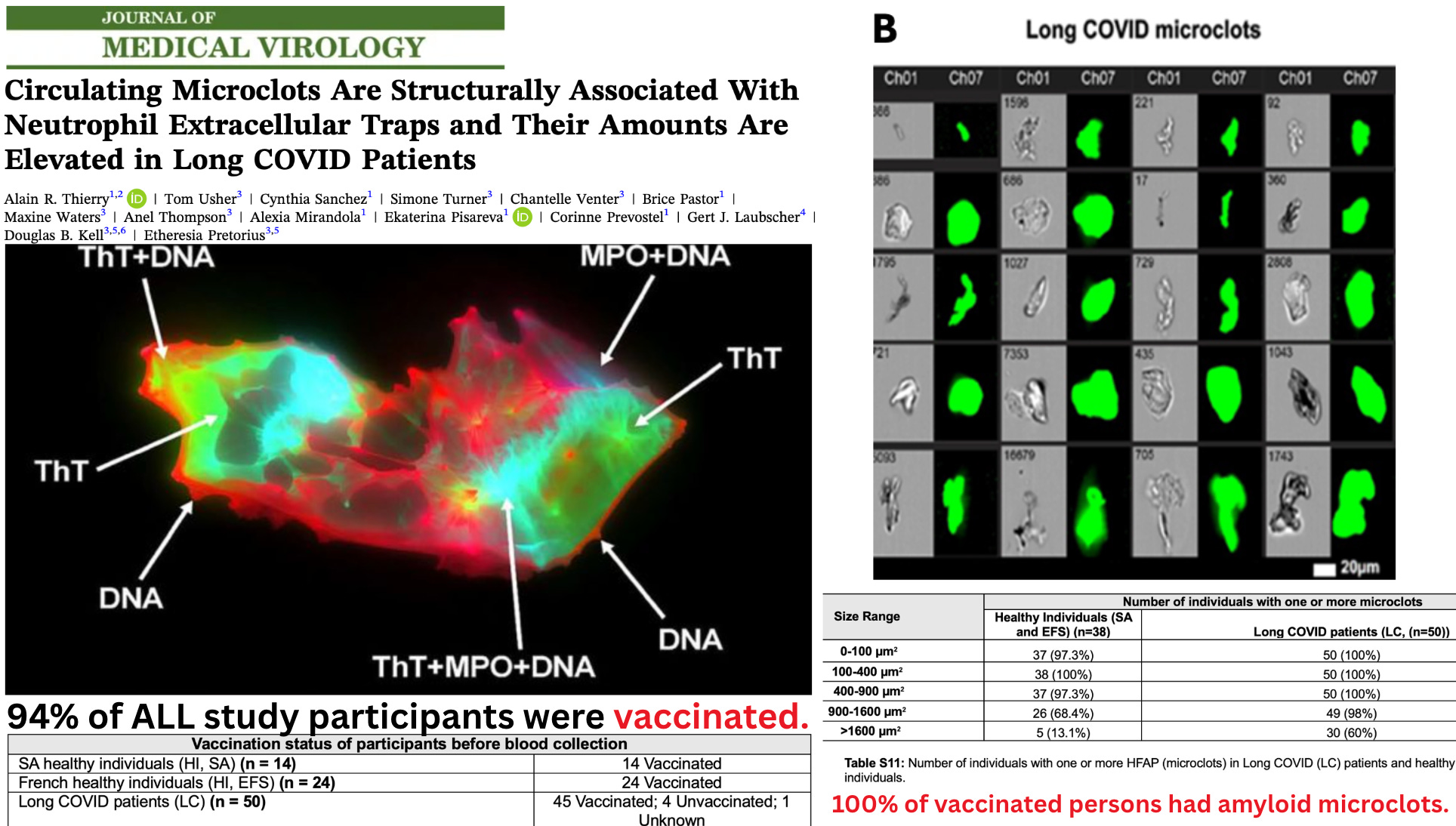
- In a cohort that was 94% vaccinated, every participant had amyloid microclots.
- The same pathology behind the large white fibrous clots now being pulled from corpses worldwide.
- The clot structures described herein exhibit abnormal morphological, histological, ultrastructural, and spectroscopic features.
- Slides: Calamari Clots Gross Anatomy.
- Nattokinase: Powerful Enzyme Prevents Heart Attack and Stroke.
- Nattokinase (pronounced nuh-TOH-kin-ayss) is an enzyme extracted and purified from a Japanese food called nattō.
- Nattō (納豆) is a traditional Japanese food made from whole soybeans that have been fermented with Bacillus subtilis var. natto.
- FIND NATTOKINASE 4U.
T=1768078253 / Human Date and time (GMT): Sat, 10th Jan, 2026, 20.50
___
BREAKING STUDY: Anomalous Amyloid Microclots Found in 100% of the COVID-19 Vaccinated
In a cohort that was 94% vaccinated, every participant had amyloid microclots —the same pathology behind the large white fibrous clots now being pulled from corpses worldwide.
Nicolas Hulscher, MPH
Nov 17, 2025
by Nicolas Hulscher, MPH
A new peer-reviewed study has quietly revealed one of the most consequential biological findings of the pandemic era — and the authors never acknowledge it: Every single vaccinated participant in the study had fibrinolysis-resistant, ThT-positive amyloid microclots circulating in their blood.
Hidden in the supplementary tables is a demographic and biochemical pattern that completely reframes the paper:
94% of all participants were vaccinated.
100% of these vaccinated individuals had amyloid microclots — including every “healthy control.”
The condition labeled “Long COVID” occurred almost entirely in a heavily vaccinated population, without any laboratory confirmation of prior SARS-CoV-2 infection. In reality, the study is observing Long VACCINE pathology, not Long COVID.
And because the authors’ own mechanistic experiments show that purified spike protein alone produces these amyloid, fibrinolysis-resistant clots, the implications are profound.
https://substackcdn.com/image/fetch/$s_!Djig!,w_1456,c_limit,f_webp,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F6064fdbe-8289-4b8f-83b9-5fcbf776405f_1928x1094.png
All individuals in the study — 100% of the vaccinated — had amyloid microclots
https://substackcdn.com/image/fetch/$s_!CY3T!,w_1456,c_limit,f_webp,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F1f082c05-b5bb-41d4-87c5-6e88c03ab7db_1680x404.png
Researchers identified microclots using Thioflavin-T (ThT), an amyloid-binding fluorogenic dye. ThT positivity was the defining criterion. A structure was only counted as a microclot if it bound ThT.
Therefore, every microclot counted in the study is, by definition, amyloidogenic.
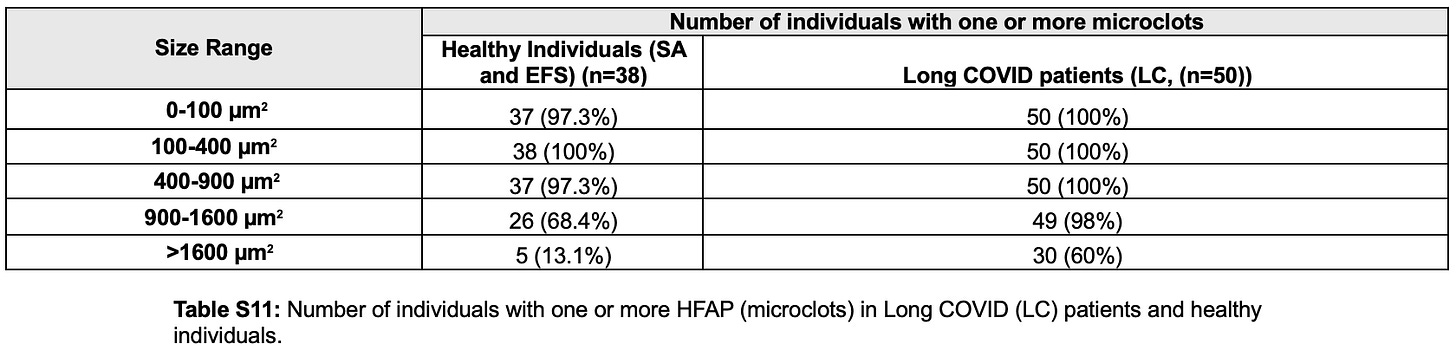
And according to Table S11, every single vaccinated participant had amyloid microclots in multiple size ranges:
Because 83 of 88 participants (94%) were vaccinated, this means:
Every vaccinated person in the study had amyloid microclots.
“Long COVID” (Long VACCINE) patients had extreme elevations in large, pathological amyloid microclots
Small amyloid microclots were present in everyone, but the pathological burden differed sharply.
According to Table S11:
98% of “Long COVID” (Long VACCINE) patients had large microclots in the 900–1600 µm² range
60% had very large microclots >1600 µm²
Total microclot burden was ~20-fold higher in “Long COVID” patients
These larger, pathogenic amyloid microclots were densely packed with:
Neutrophil extracellular traps (NETs)
Myeloperoxidase
Neutrophil elastase
Extracellular DNA
Misfolded amyloid fibrin
COVID-19 infection was never verified
Despite positioning the results as a hallmark of “Long COVID,” none of the participants were confirmed to have had SARS-CoV-2 infection. The study performed:
no antibody testing
no PCR
no sequencing
no neutralizing antibody assays
Long COVID status was assigned purely via symptoms and clinician impression. There is no evidence in the study that any participant was biologically positive for prior infection.
Thus, the clotting abnormalities cannot be attributed specifically to infection, but rather to vaccination.
Spike protein alone produced identical amyloid microclots
In a mechanistic experiment, the authors added purified spike protein to fibrinogen.
This single intervention produced:
insoluble, ThT-positive amyloid microclots
misfolded fibrin structures identical to those in patient samples
fibrinolysis-resistant aggregates compatible with vessel obstruction
The authors confirmed that Spike protein directly induces amyloid microclot formation, corroborating previous studies.
Explains prevalent white fibrous clots found in the dead
The study’s core findings — 100% amyloid microclots in vaccinated individuals and direct spike-induced amyloid fibrin formation — offer a clear mechanism for the large, rubbery white fibrous clots increasingly reported in deceased individuals since 2021.
At the 2025 Tennessee Funeral Directors Association (TFDA) convention, former USAF Major Tom Haviland conducted the first state-level survey of embalmers:
64% reported white fibrous clots in 2025
Found in 17% of all bodies
70% observed widespread microclotting (“coffee-grounds blood”)
39% reported rising infant deaths (+14%)
BREAKING: Tennessee Funeral Directors Association Confirms White Fibrous Clots Are Real and Prevalent

https://substackcdn.com/image/fetch/$s_!u72N!,w_1300,h_650,c_fill,f_webp,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-video.s3.amazonaws.com%2Fvideo_upload%2Fpost%2F166074998%2F755304bd-f03d-467f-b338-a324154f4cec%2Ftranscoded-07729.png
By Nicolas Hulscher, MPH
Forensic analysis by Kevin W. McCairn, PhD et al shows that these postmortem clots:
are amyloidogenic fibrin aggregates, not normal thrombi
exhibit β-sheet structures (ThT-positive)
are protease-resistant, rubbery, and fibrous
have dense fibrillar ultrastructure on SEM
contain human genetic material
and show preliminary plasmid/spike-associated markers

https://substackcdn.com/image/fetch/$s_!nWuL!,w_1456,c_limit,f_webp,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2Fa220e719-856a-4643-a440-1954daf837d6_1456x1119.webp
These characteristics match exactly the pathological microclots described in the new study — only at a later, aggregated, end-stage form.
The progression is biologically straightforward:
Spike exposure (infection or mRNA vaccination)
Amyloid microclots form — present in 100% of vaccinated subjects
Large, NET-rich, fibrinolysis-resistant clots accumulate (20× higher in Long vaccine patients)
These merge into massive, rubbery, white fibrous intravascular clots
This new study documents the early and intermediate stages in the living; Haviland’s surveys and McCairn’s analysis reveals the final stage in the dead.
Conclusions
Although the authors frame their findings as “Long COVID,” the underlying data reveal something far more consequential:
100% of vaccinated participants had amyloid microclots.
Large, fibrinolysis-resistant amyloid microclots were concentrated in the Long vaccine group.
No participant had laboratory-confirmed SARS-CoV-2 infection.
Spike protein alone produced identical amyloid microclots in vitro.
With 94% vaccination uptake, the biological signal is overwhelmingly linked to spike exposure in a vaccinated population.
These findings carry serious public-health implications:
Every vaccinated individual in the study showed early-stage amyloid microclots, raising alarms about cumulative vascular injury across the entire globe.
The pathology mirrors the large white fibrous clots now documented by embalmers and forensic analysts.
And critically:
The CDC and federal public-health agencies must finally do their job and launch an immediate, transparent investigation into these findings.
Failing to intestigate the white fibrous clot situation constitutes a dereliction of duty.
Any platform delivering spike protein into human circulation must be immediately banned for human use.
Epidemiologist and Foundation Administrator, McCullough Foundation
Support our mission: mcculloughfnd.org
Please consider following both the McCullough Foundation and my personal account on X (formerly Twitter) for further content.
_
FOCAL POINTS (Courageous Discourse) is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber.
SOURCE:
https://www.thefocalpoints.com/p/breaking-study-anomalous-amyloid?_x_tr_hist=true
___
Cadaver "Calamari" Amyloidogenic Fibrin Aggregates
Spike Protein Pathology from Cadavers Exposed to Bioengineered SARS-CoV-2
Kevin W. McCairn Ph.D.May 10, 2025
Kevin W. McCairn, Ph.D., Kevin McKernan Ph.D., Shojiro Kato M.D., Charles Rixey retired (USMC-CBRN)
Abstract
This report presents a preliminary forensic analysis of anomalous fibrin-like aggregates recovered from postmortem human cadaveric samples. Through a combination of gross morphological inspection, cryosection histology, fluorescent staining, scanning electron microscopy (SEM), elemental analysis (EDX), real-time PCR, Raman spectroscopy, and Real-Time Quaking-Induced Conversion (RT-QuIC), the samples were examined for biochemical and ultrastructural features associated with amyloidogenic and protease-resistant fibrin formation. Findings suggest that the clot samples exhibit hallmarks of abnormal protein aggregation consistent with pathological fibrin remodeling, including enhanced autofluorescence, beta-sheet rich domains, dense fibrillar ultrastructure, and spectral anomalies. PCR confirmed the human origin of the tissues, and preliminary evidence of molecular markers associated with recombinant spike protein exposure (SV40 & Ori) was observed. Limitations of provenance, sample control, and chain-of-custody are acknowledged, and further investigation is recommended to establish clinical, pathological, and etiological relevance.
Introduction
Recent reports have drawn attention to the presence of large, rubbery, white fibrin-like clots recovered from postmortem human vasculature. These structures have been described anecdotally by embalmers and morticians and have raised questions regarding their origin, composition, and potential pathological significance. In response, this investigative effort sought to apply rigorous analytical methodologies to determine whether these samples represent a known thrombotic phenotype or reflect novel features associated with emergent pathologies.
It is now broadly recognized that SARS-CoV-2 infection can exert systemic effects beyond its respiratory tropism, with evidence pointing to persistent coagulopathy and fibrin dysregulation. Of particular concern is the potential of the viral spike protein—whether introduced via infection or recombinant delivery platforms such as LNP-mRNA vaccines—to catalyze the formation of amyloid-like fibrin clots. Prior studies have demonstrated that spike exposure can induce fibrin(ogen) conformational changes that resist fibrinolysis and exhibit beta-sheet structures akin to amyloid fibrils.
This report investigates whether the cadaver-derived clots under study bear the morphological and biochemical hallmarks of such aberrant fibrin. Key research questions include: Are the structures of human origin? Do they exhibit features consistent with amyloidogenesis? Can they be differentiated from conventional fibrin emboli or white clots found in known medical conditions?
Through a multidisciplinary forensic approach, this document catalogs the physical and biochemical attributes of the clot specimens, aiming to provide a transparent, methodologically sound foundation for independent review. Given the limitations of the sampling context and absence of complete clinical background, the findings presented here are exploratory and intended to prompt further controlled research.
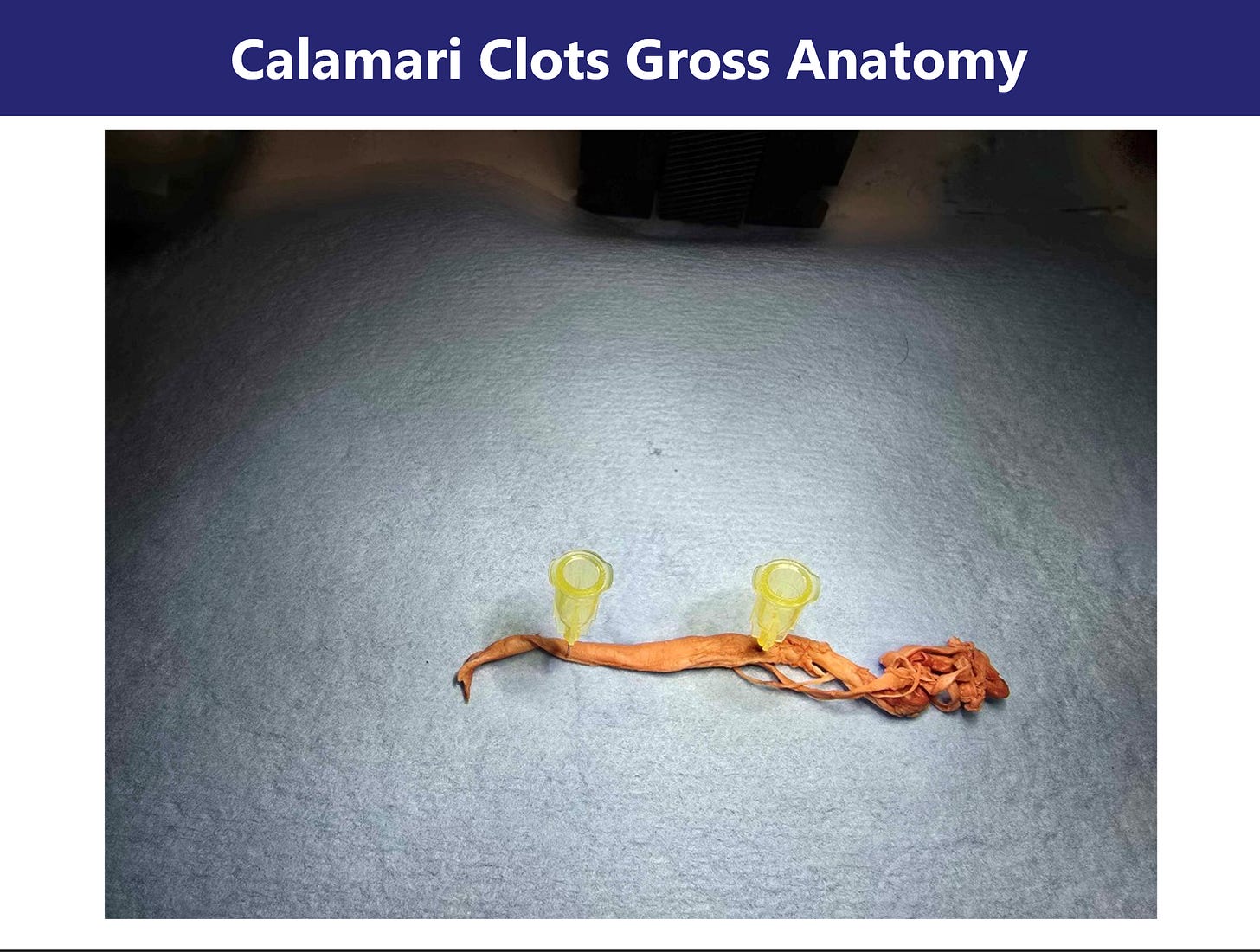
Section 1: Gross Morphology of Clot Structures Image: Slides 1–5
Initial macroscopic inspection of the cadaver-derived clot specimens revealed strikingly anomalous morphology. The samples appear rubbery, fibrous, and frequently coiled or banded—bearing superficial resemblance to marine calamari. Unlike conventional thrombi, these aggregates lack the red-brown coloration and laminar erythrocyte-rich stratification commonly observed in postmortem clots.
Images collected at 4K resolution under dissecting microscope conditions show that the structures retain a solid, tensile integrity. The specimens resist tearing, do not collapse under moderate compression, and maintain structural coherence when manipulated, suggesting a composition distinct from typical fibrin gels. Internal cross-sections show a dense, layered architecture without clear vascular lumens, further differentiating them from vessel-associated thrombi.
These morphological traits suggest either advanced cross-linking within a protein matrix or a structurally aberrant form of polymerized fibrin. The preserved integrity and resistance to manipulation raise the possibility of amyloidogenic remodeling—a hypothesis explored in the following sections through microscopic and spectroscopic analysis.
Section 2: Histology and Fluorescent Imaging of Cryosections Image: Slides 6–11
Tissue samples were cryosectioned at 20 µm thickness and subjected to light and fluorescence microscopy at both 4X and 40X magnification. Hematoxylin-free imaging reveals a heterogeneous, fibrous internal structure with numerous cavities and layered fibrillar regions.
Fluorescence analysis prior to staining revealed strong and uniform intrinsic autofluorescence. Under blue-light excitation, green emission was observed; excitation in the green channel induced red fluorescence. This broad-spectrum response is highly atypical and suggests a high degree of intrinsic molecular ordering or aromatic residue stacking. Such behavior is consistent with cross-linked protein networks or amyloid-like interactions involving tyrosine, tryptophan, or phenylalanine residues.
Thioflavin T (ThT) staining was used to assess the presence of beta-sheet rich amyloid domains. Comparative imaging before and after ThT application revealed discrete "polka-dot" fluorescent foci, as well as diffuse interspersed fluorescence. These data indicate that amyloidogenic stacking is localized but not homogeneous, suggesting microclot heterogeneity within the sample.
Section 3: Scanning Electron Microscopy (SEM) and Elemental Composition via EDX Image: Slides 12–17
Cryosections of 5 µm thickness were prepared for SEM. At low magnification (25X), overall morphology was assessed. Higher magnifications (up to 5000X) revealed a dense reticular meshwork of fibrillar aggregates.
These fibrils display nodular topography and branching interconnections, consistent with pathological protein assembly. Notably, a rotational twist and lateral aggregation features were observed, hallmarks of amyloid fibrin architecture. These ultrastructural findings support the hypothesis that the observed material is not simple polymerized fibrin but instead a protease-resistant, misfolded protein aggregate.
Elemental mapping via EDX revealed high abundance of carbon, nitrogen, oxygen, and sulfur—consistent with proteinaceous material. No significant signal was detected for heavy metals (e.g., Fe, Zn, Cu), ruling out mineral-based aggregation or contamination. The absence of inorganic nucleation points supports an endogenous biochemical origin.
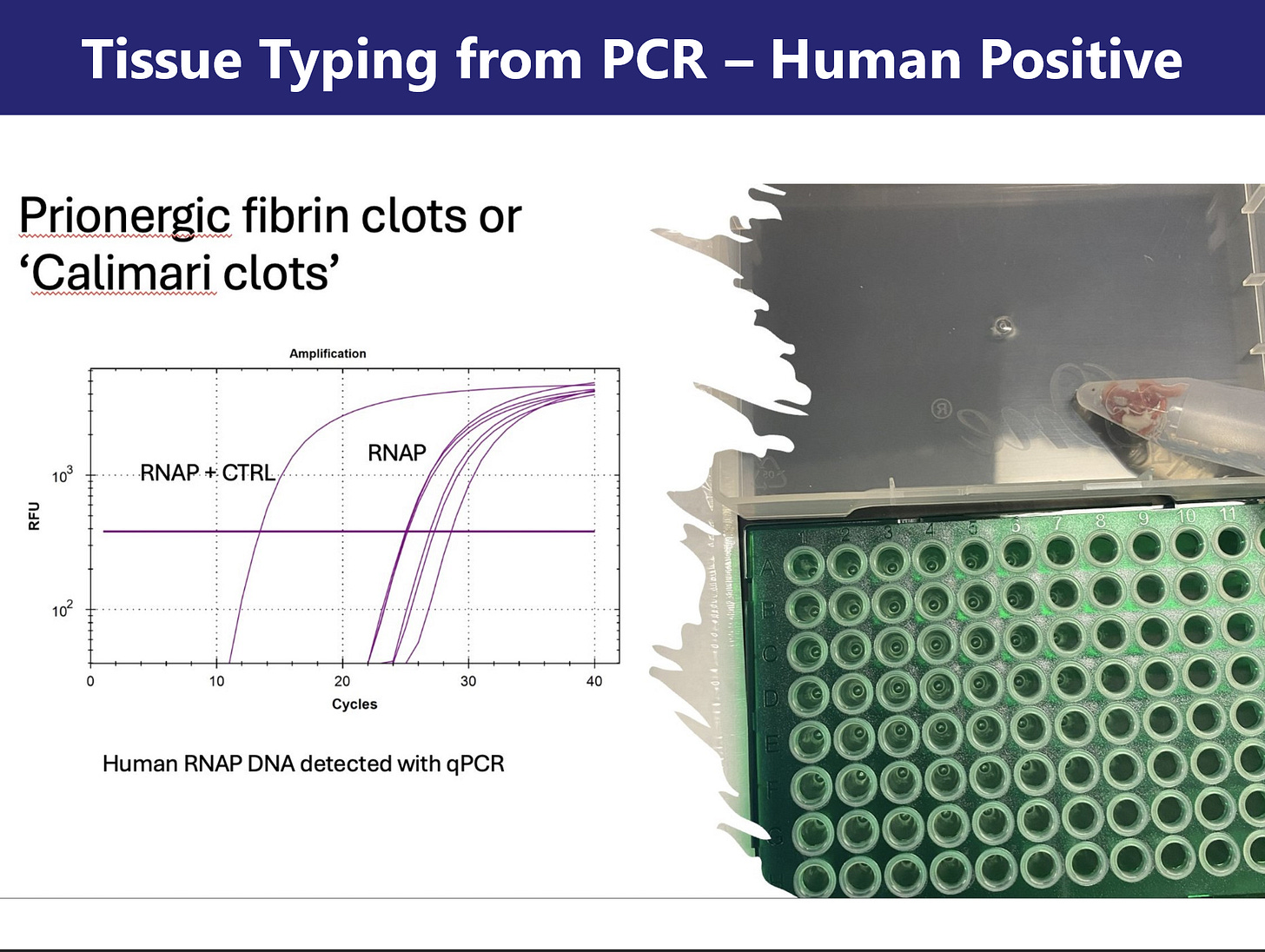
Section 4: PCR and Raman Spectroscopy for Tissue and Molecular Signature Image: Slides 18-20
PCR using primers for the human RNaseP transcript confirmed that the clot-derived samples were of human origin. Additional assays targeting spike protein coding sequences and plasmid-related markers (SV40, Ori) revealed late-cycle amplification, consistent with trace residual presence of recombinant vaccine components. These findings are preliminary and require cautious interpretation.
Raman spectroscopy revealed a major spectral peak at ~1720 cm⁻¹, diverging from the canonical amyloid beta-sheet signature near 1670 cm⁻¹. This spectral upshift may reflect altered secondary structure, ester linkage formation, or protonation of acidic residues. The results point to an atypical fibril composition that may represent a novel polymorph or hybrid aggregate class.
Section 5: RT-QuIC Seeding Activity in Plasma Extracts
RT-QuIC analysis was used to assess seeding potential of the clot-associated material. 3D bar plot visualization of fluorescence intensity across a 96-well plate revealed elevated ThT signal in experimental wells, consistent with fibril formation, when challenged against human plasma.
However, background signal, handling artifacts, and absence of dilution series limit interpretation. Without kinetic rate modeling and control seeding, the data remain suggestive but inconclusive for prion-like activity.
Conclusion
The clot structures described herein exhibit abnormal morphological, histological, ultrastructural, and spectroscopic features. Their dense fibrillar architecture, autofluorescence, ThT reactivity, spectral shifts, and preliminary RT-QuIC activity suggest amyloidogenic remodeling of fibrin under unknown conditions.
While the findings are not definitive, they raise substantial biosafety and pathophysiological questions that warrant immediate, controlled follow-up. The uncertain provenance and unstandardized collection methodology underscore the need for independent replication with verified chain-of-custody.
In the current context of vaccine-induced spike protein exposure and post-infectious complications, these results may point to a novel or under-recognized pathology. As such, they constitute a call for interdisciplinary scientific inquiry, clinical vigilance, and transparent investigation.
Slides


















This Substack is reader-supported. To receive new posts and support my work, consider becoming a free or paid subscriber.
SOURCE:
https://kevinwmccairnphd282302.substack.com/p/cadaver-calamari-amyloidogenic-fibrin
___
eof
